Comparable A1C reduction to Humalog
ADMELOG® demonstrated non-inferiority vs Humalog and a non–US-approved insulin lispro 100 Units/mL in SORELLA 2, a 26-week head-to-head trial in adult patients with type 2 diabetes1

ADMELOG
+
Lantus®

n=253
Humalog
+
Lantus

n=252

Adjusted mean difference of −0.06% (non-inferiority margin of 0.3%; N=505; 95% CI: −0.209 to 0.091)
STUDY RESULT:
Patients treated with ADMELOG demonstrated non-inferiority in A1C change from baseline compared with patients treated with Humalog (−0.86% and −0.80%, respectively), at a prespecified non-inferiority margin of 0.3% (adjusted mean difference of −0.06%; 95% CI: −0.209 to 0.091).1
SORELLA 2 STUDY DESIGN:
A 26-week, open-label, active-controlled study evaluated the glucose lowering effect of ADMELOG plus insulin glargine, 100 Units/mL, compared to that of Comparator (another insulin lispro product, 100 Units/mL, or a non–US-approved insulin lispro, 100 Units/mL), plus insulin glargine, 100 Units/mL. A total of 505 patients with T2DM treated with insulin glargine 100 Units/mL and rapid-acting mealtime insulin analogs participated in the study. Patients were randomized to ADMELOG (n=253) or Comparator (n=252). ADMELOG or Comparator was administered by subcutaneous injection immediately prior to meals. The primary endpoint was a change in A1C from baseline to week 26 (non-inferiority margin of 0.3%; Cl of −0.209 to 0.091).1,2
Incidence of adverse events for ADMELOG and Humalog
Incidence of any or severe hypoglycemia for ADMELOG, Humalog, and a non–US-approved insulin lispro 100 Units/mL in SORELLA 2, a 26-week head-to-head trial in adult patients with type 2 diabetes1,2

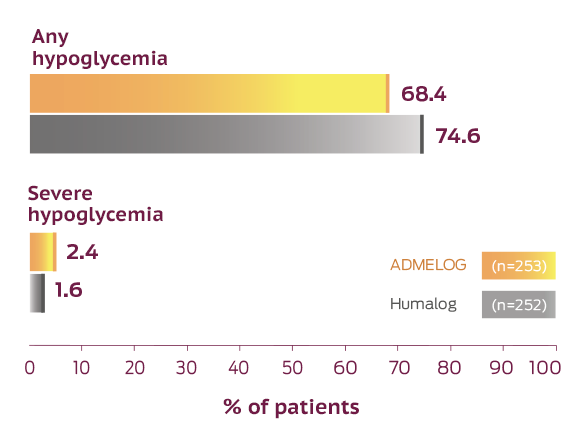
In this trial, hypoglycemia was the only adverse reaction occurring in ≥5% of patients treated with ADMELOG.1
Hypoglycemia is the most common adverse event for insulin-containing therapies.
This trial was not designed to evaluate the relative safety between ADMELOG and Humalog, and Comparator adverse event rates are not an adequate basis for comparison of rates between the products. Rates of hypoglycemia depend on numerous factors; therefore, comparing ADMELOG with other products is not appropriate. The rates of hypoglycemia may not be representative of rates that will occur in clinical practice.1
In the ADMELOG trials, severe hypoglycemia was defined as an event requiring assistance of another person to actively administer carbohydrate, glucagon, or other resuscitative actions. Documented symptomatic hypoglycemia was defined as an event with typical symptoms of hypoglycemia accompanied by a self-monitored glucose value equal to or less than 70 mg/dL.1,2
A1C reduction with ADMELOG was comparable to Humalog
ADMELOG demonstrated non-inferiority vs Humalog and a non–US-approved insulin lispro 100 Units/mL in SORELLA 1, a 26-week head-to-head trial in adult patients with type 1 diabetes1

ADMELOG
+
Lantus

n=253
Humalog
+
Lantus
n=254

Adjusted mean difference of 0.06% (non-inferiority margin of 0.3%; N=507; 95% CI: −0.086 to 0.201)
STUDY RESULT:
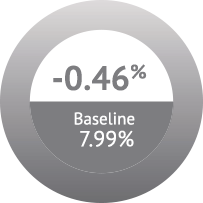
Patients treated with ADMELOG demonstrated non-inferiority in A1C change from baseline compared with patients treated with Humalog (−0.40% and −0.46%, respectively), at a prespecified non-inferiority margin of 0.3% (adjusted mean difference of 0.06%; N=507; 95% CI: −0.086 to 0.201).1,3,4
SORELLA 1 STUDY DESIGN:
A 26-week, open-label, active-controlled study evaluated the glucose lowering effect of ADMELOG plus insulin glargine, 100 Units/mL, compared to that of Comparator (another insulin lispro product, 100 Units/mL, or a non–US-approved insulin lispro, 100 Units/mL), plus insulin glargine, 100 Units/mL. A total of 507 patients with T1DM treated with insulin glargine 100 Units/mL and rapid-acting mealtime insulin analogs participated in the study. Patients were randomized to ADMELOG (n=253) or Comparator (n=254). ADMELOG or Comparator was administered by subcutaneous injection immediately prior to meals. The primary endpoint was a change in A1C from baseline to week 26 (non-inferiority margin of 0.3%; Cl of −0.086 to 0.201).1,3,4
Incidence of adverse events for ADMELOG and Humalog
Incidence of any or severe hypoglycemia for ADMELOG, Humalog, and a non–US-approved insulin lispro 100 Units/mL in SORELLA 1, a 26-week head-to-head trial in adult patients with type 1 diabetes, followed by a 26-week safety extension1,3,4

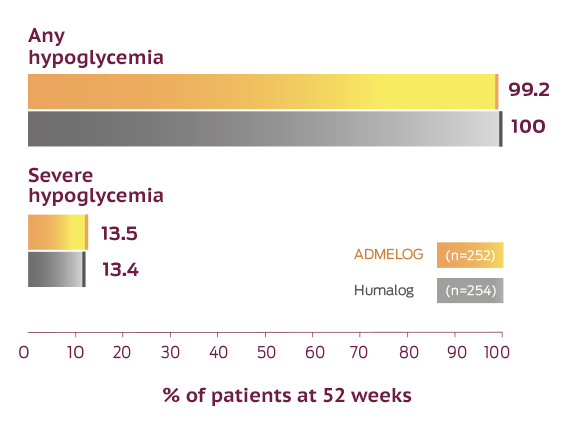
Hypoglycemia is the most common adverse event for insulin-containing therapies.
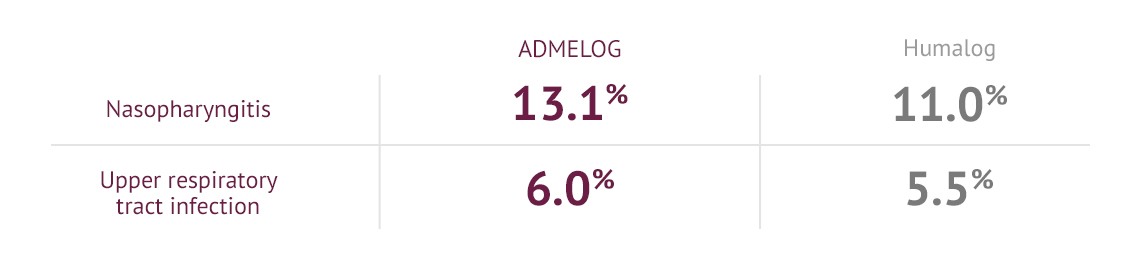
Common adverse reactions at 52 weeks (other than hypoglycemia) occurring in ≥5% of adult patients with type 1 diabetes treated with ADMELOG1,3,4
This trial was not designed to evaluate the relative safety between ADMELOG and Humalog, and Comparator adverse event rates are not an adequate basis for comparison of rates between the products. Rates of hypoglycemia depend on numerous factors; therefore, comparing ADMELOG with other products is not appropriate. The rates of hypoglycemia may not be representative of rates that will occur in clinical practice.1
In the ADMELOG trials, severe hypoglycemia was defined as an event requiring assistance of another person to actively administer carbohydrate, glucagon, or other resuscitative actions. Documented symptomatic hypoglycemia was defined as an event with typical symptoms of hypoglycemia accompanied by a self-monitored glucose value equal to or less than 70 mg/dL.1,4


Dosing & Administration
No dose calculation needed when transitioning from Humalog1†
†Dose adjustment may be needed when switching from another fast-acting insulin or mealtime insulin to ADMELOG.1
Indication for ADMELOG® (insulin lispro injection) 100 Units/mL
ADMELOG is a rapid-acting human insulin analog indicated to improve glycemic control in adults with type 2 diabetes and adults and children (3 years and older) with type 1 diabetes.
Important Safety Information
Important Safety Information
CONTRAINDICATIONS
ADMELOG is contraindicated during episodes of hypoglycemia, and in patients with hypersensitivity to insulin lispro or to any of its excipients.
WARNINGS AND PRECAUTIONS
Insulin pens and needles must never be shared between patients, even if the needle is changed. Do NOT reuse needles.
Monitor blood glucose in all patients treated with insulin. Modify insulin regimens only under medical supervision. Changes in insulin regimen including, strength, manufacturer, type, injection site or method of administration may result in the need for a change in insulin dose or an adjustment in concomitant oral antidiabetic treatment. Changes in insulin regimen may result in hyperglycemia or hypoglycemia.
Repeated insulin injections into areas of lipodystrophy or localized cutaneous amyloidosis may result in hyperglycemia; sudden change in the injection site (to unaffected area) has been reported to result in hypoglycemia. Advise patients to rotate injection site to unaffected areas and closely monitor for hypoglycemia.
Hypoglycemia is the most common adverse reaction associated with insulins, including ADMELOG, and may be life-threatening.
Accidental mix-ups between basal insulin products and other insulins, particularly rapid-acting insulins, have been reported. To avoid medication errors between ADMELOG and other insulins, instruct patients to always check the insulin label before each injection.
Severe, life-threatening, generalized allergy, including anaphylaxis, can occur with insulin products, including ADMELOG. If hypersensitivity reactions occur, discontinue ADMELOG, treat per standard of care and monitor until symptoms and signs resolve.
All insulin products, including ADMELOG, can cause hypokalemia. Untreated hypokalemia may cause respiratory paralysis, ventricular arrhythmia, and death. Monitor potassium levels in patients at risk for hypokalemia.
Fluid retention and heart failure can occur with concomitant use of thiazolidinediones (TZDs) with insulin. Observe for signs and symptoms of heart failure. Consider dosage reduction or discontinuation of TZD if heart failure occurs.
ADVERSE REACTIONS
Adverse reactions associated with ADMELOG include hypoglycemia, hypokalemia, allergic reactions, injection site reactions, lipodystrophy, pruritus, rash, weight gain, and peripheral edema.
DRUG INTERACTIONS
Certain drugs may affect glucose metabolism, requiring insulin dose adjustment and close monitoring of blood glucose. The signs of hypoglycemia may be reduced in patients taking anti-adrenergic drugs (eg, beta-blockers, clonidine, guanethidine, and reserpine).
IMPORTANT SAFETY INFORMATION FOR ADMELOG® (INSULIN LISPRO INJECTION) SOLOSTAR®
ADMELOG SoloStar is a disposable single-patient-use prefilled insulin pen. To help ensure an accurate dose each time, patients should follow the steps in the Instruction Leaflet accompanying the pen; otherwise they may not get the correct amount of insulin, which may affect their blood glucose levels.
IMPORTANT SAFETY INFORMATION FOR ADMELOG® (INSULIN LISPRO INJECTION) USE IN PUMP
Pump failure or insulin infusion set, or insulin degradation can rapidly lead to hyperglycemia and ketoacidosis. Prompt identification and correction of the cause is necessary. Interim subcutaneous injections with ADMELOG may be required. Patients using a pump must be trained to administer insulin by injection and have alternate insulin therapy available in case of pump failure.
Click here for Full Prescribing Information.
Click here for information on Sharps Medical Waste Disposal.
Click here to learn more about Sanofi's commitment to fighting counterfeit drugs.
*Eligibility Restrictions & Offer Terms for your Patients:
Insulins Valyou Savings Program: This savings program is not insurance. For a complete list of participating brands, products, and National Drug Codes (NDCs) Click Here. This offer is not valid for prescriptions covered by or submitted for reimbursement, in whole or in part, under Medicare, Medicaid, VA, DOD, TRICARE, similar federal or state programs, including any state pharmaceutical programs. If you have an Affordable Care (Health Care Exchange) plan, you may still be qualified to receive and use this savings card. Please note: the Federal Employees Health Benefits (FEHB) Program is not a federal or state government health care program for purposes of the savings program. Void where prohibited by law. The Savings Program applies to the cost of medication. There are other relevant costs associated with overall treatment. Sanofi reserves the right to rescind, revoke, terminate, or amend this offer, eligibility, and terms of use at any time without notice. Upon registration, patients will receive all program details. For questions regarding your eligibility or benefits, or if you wish to discontinue your participation, call the Insulins Valyou Savings Program at (833) 813-0190(833) 813-0190 (8:00 am-8:00 pm EST, Monday-Friday).
Sanofi Insulins Co-pay Savings Program: This savings program is not insurance. For a complete list of participating brands, products, and National Drug Codes (NDCs) Click Here. This offer is not valid for prescriptions covered by or submitted for reimbursement, in whole or in part, under Medicare, Medicaid, VA, DOD, TRICARE, similar federal or state programs, including any state pharmaceutical programs. If you have an Affordable Care (Health Care Exchange) plan, you may still be qualified to receive and use this savings card. Please note: the Federal Employees Health Benefits (FEHB) Program is not a federal or state government health care program for purposes of the savings program. Void where prohibited by law. There are other relevant costs associated with overall treatment. Sanofi reserves the right to rescind, revoke, terminate, or amend this offer, eligibility, and terms of use at any time without notice. Upon registration, patients will receive all program details. For questions regarding your eligibility or benefits, or if you wish to discontinue your participation, call the Sanofi Insulins Co-pay Savings Program at (866) 255-8661(866) 255-8661 (8:00 am-8:00 pm EST, Monday-Friday).
References:
1. ADMELOG Prescribing Information.
2. Derwahl K-M, Bailey TS, Wernicke-Panten K, Ping L, Pierre S. Diabetes Technol Ther. 2018;20(1):49-58.
3. Data on file (SORELLA 1 study, October 2016).
4. Garg SK, Wernicke-Panten K, Rojeski M, Pierre S, Kirchhein Y, Jedynasty K. Diabetes Technol Ther. 2017;19(9):516-526.
Important Safety Information
CONTRAINDICATIONS
ADMELOG is contraindicated during episodes of hypoglycemia, and in patients with hypersensitivity to insulin lispro or to any of its excipients.
WARNINGS AND PRECAUTIONS
Insulin pens and needles must never be shared between patients, even if the needle is changed. Do NOT reuse needles.
Monitor blood glucose in all patients treated with insulin. Modify insulin regimens only under medical supervision. Changes in insulin regimen including, strength, manufacturer, type, injection site or method of administration may result in the need for a change in insulin dose or an adjustment in concomitant oral antidiabetic treatment. Changes in insulin regimen may result in hyperglycemia or hypoglycemia.
Repeated insulin injections into areas of lipodystrophy or localized cutaneous amyloidosis may result in hyperglycemia; sudden change in the injection site (to unaffected area) has been reported to result in hypoglycemia. Advise patients to rotate injection site to unaffected areas and closely monitor for hypoglycemia.
Hypoglycemia is the most common adverse reaction associated with insulins, including ADMELOG, and may be life-threatening.
Accidental mix-ups between basal insulin products and other insulins, particularly rapid-acting insulins, have been reported. To avoid medication errors between ADMELOG and other insulins, instruct patients to always check the insulin label before each injection.
Severe, life-threatening, generalized allergy, including anaphylaxis, can occur with insulin products, including ADMELOG. If hypersensitivity reactions occur, discontinue ADMELOG, treat per standard of care and monitor until symptoms and signs resolve.
All insulin products, including ADMELOG, can cause hypokalemia. Untreated hypokalemia may cause respiratory paralysis, ventricular arrhythmia, and death. Monitor potassium levels in patients at risk for hypokalemia.
Fluid retention and heart failure can occur with concomitant use of thiazolidinediones (TZDs) with insulin. Observe for signs and symptoms of heart failure. Consider dosage reduction or discontinuation of TZD if heart failure occurs.
ADVERSE REACTIONS
Adverse reactions associated with ADMELOG include hypoglycemia, hypokalemia, allergic reactions, injection site reactions, lipodystrophy, pruritus, rash, weight gain, and peripheral edema.
DRUG INTERACTIONS
Certain drugs may affect glucose metabolism, requiring insulin dose adjustment and close monitoring of blood glucose. The signs of hypoglycemia may be reduced in patients taking anti-adrenergic drugs (eg, beta-blockers, clonidine, guanethidine, and reserpine).
IMPORTANT SAFETY INFORMATION FOR ADMELOG® (INSULIN LISPRO INJECTION) SOLOSTAR®
ADMELOG SoloStar is a disposable single-patient-use prefilled insulin pen. To help ensure an accurate dose each time, patients should follow the steps in the Instruction Leaflet accompanying the pen; otherwise they may not get the correct amount of insulin, which may affect their blood glucose levels.
IMPORTANT SAFETY INFORMATION FOR ADMELOG® (INSULIN LISPRO INJECTION) USE IN PUMP
Pump failure or insulin infusion set, or insulin degradation can rapidly lead to hyperglycemia and ketoacidosis. Prompt identification and correction of the cause is necessary. Interim subcutaneous injections with ADMELOG may be required. Patients using a pump must be trained to administer insulin by injection and have alternate insulin therapy available in case of pump failure.
Click here for Full Prescribing Information.
Click here for information on Sharps Medical Waste Disposal.
Click here to learn more about Sanofi's commitment to fighting counterfeit drugs.
*Eligibility Restrictions & Offer Terms for your Patients:
Insulins Valyou Savings Program: This savings program is not insurance. For a complete list of participating brands, products, and National Drug Codes (NDCs) Click Here. This offer is not valid for prescriptions covered by or submitted for reimbursement, in whole or in part, under Medicare, Medicaid, VA, DOD, TRICARE, similar federal or state programs, including any state pharmaceutical programs. If you have an Affordable Care (Health Care Exchange) plan, you may still be qualified to receive and use this savings card. Please note: the Federal Employees Health Benefits (FEHB) Program is not a federal or state government health care program for purposes of the savings program. Void where prohibited by law. The Savings Program applies to the cost of medication. There are other relevant costs associated with overall treatment. Sanofi reserves the right to rescind, revoke, terminate, or amend this offer, eligibility, and terms of use at any time without notice. Upon registration, patients will receive all program details. For questions regarding your eligibility or benefits, or if you wish to discontinue your participation, call the Insulins Valyou Savings Program at (833) 813-0190(833) 813-0190 (8:00 am-8:00 pm EST, Monday-Friday).
Sanofi Insulins Co-pay Savings Program: This savings program is not insurance. For a complete list of participating brands, products, and National Drug Codes (NDCs) Click Here. This offer is not valid for prescriptions covered by or submitted for reimbursement, in whole or in part, under Medicare, Medicaid, VA, DOD, TRICARE, similar federal or state programs, including any state pharmaceutical programs. If you have an Affordable Care (Health Care Exchange) plan, you may still be qualified to receive and use this savings card. Please note: the Federal Employees Health Benefits (FEHB) Program is not a federal or state government health care program for purposes of the savings program. Void where prohibited by law. There are other relevant costs associated with overall treatment. Sanofi reserves the right to rescind, revoke, terminate, or amend this offer, eligibility, and terms of use at any time without notice. Upon registration, patients will receive all program details. For questions regarding your eligibility or benefits, or if you wish to discontinue your participation, call the Sanofi Insulins Co-pay Savings Program at (866) 255-8661(866) 255-8661 (8:00 am-8:00 pm EST, Monday-Friday).



